How to Charge a Gel Battery
BATTERY APPLICATIONS
Data safe battery: no leakage on gel battery terminal, ensure using in safe and reliable.
Maintenance free battery: due to all internal generated gas restore to water, do not need water replenishment.
Exhaust air system: it can exhaust excess gas and make air pressure up to normal range when gel motorcycle battery overcharges and internal pressure is over high, this time safe valve will close by itself, so there will be not additional gas accumulate. PRODUCT DESCRIPTION.
No free acid: special separator adsorb electrolyte, so there is no free acid inside lead acid battery, then vrla battery can be installed in various position.
CHEMICAL REACTIONINVRLA BATTERY SA FOLLOWS
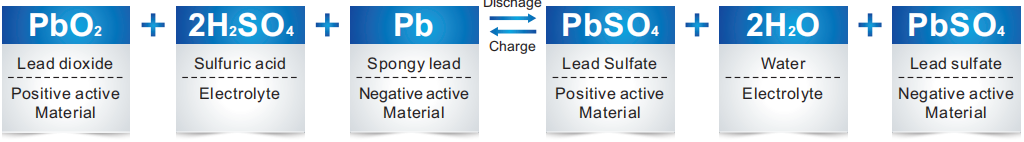
While gel battery is discharged, the concentration of sulfuric acid is gradually decreased and lead sulfate is formed under the reaction between lead dioxide of positive electrode, spongy lead of negative electrode and the sulfuric acid in the electrolyte.
While charging, lead sulfate in the positive and negative electrode is transformed to lead dioxide and spongy lead, and with the separation of sulfuric ions, the concentration of sulfuric acid will increase.
During the last charging period of traditional lead acid battery, water is consumed by the reaction of hydrogen evolution. So it requires compensation of water. With the application of moist spongy lead, it promptly reacts with oxygen, which effectively controls the decrease of water.
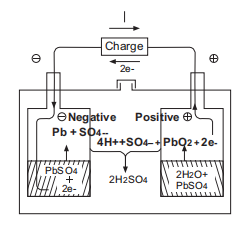
It is same as the traditional gel batteries from the beginning of charge to before the final stage, but when it is over-charged and in the last period of charge, the electric power will start to decompose water, negative electrode will be in discharge condition because oxygen from the positive plate reacts with spongy lead of negative plate and sulfuric acid of electrolyte. That restrains the hydrogen evolution on the negative plates. The part of negative electrode in discharge condition will transform to spongy lead while charging.
The quantity of spongy lead formed from charging equals to the quantity of sulfate lead as the result of absorbing the oxygen from positive electrode, which keeps the balance of negative electrode, and also make it possible to seal 12v 12ah gel cell battery. Reaction after the final stage of charge and chemical equation as below:
Fig.3:Reaction From Beginning Of Charge To Before the Final Stage
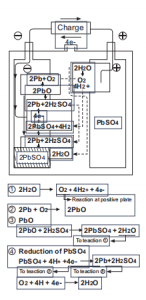
why choose us?
1. 100% Pre-delivery inspection to ensure stable quality and reliable performance.
2. Pb-Ca grid alloy VRLA battery plate, low water loss, and stable quality low self-discharge rate.
3. Low internal resistance, good high rate discharge performance.
4. The flooded electrolyte design, sufficient electrolyte, high over-charge/over-discharge resistance.
5. Excellence high-and-low temperature performance, working temperature ranging from -25℃ to 50℃.
6. Design float service life: 3-5 years.
Post time: Apr-07-2022

