Indlela yokuTshaja ibhetri yeGel
IZICELO ZEBHETRI
Ibhetri ekhuselekileyo yedatha:akukho kuvuza kwi-terminal yebhetri yejeli, qinisekisa ukusebenzisa ngokukhuselekileyo nangokuthembekileyo.
Ukugcinwa kwebhetri yasimahla:ngenxa yayo yonke igesi eyenziwe yangaphakathi ebuyiselwa emanzini, ayifuni ukuzaliswa kwamanzi.
Inkqubo yokukhupha umoya:inokukhupha igesi engaphezulu kwaye yenze uxinzelelo lomoya ukuya kuluhlu oluqhelekileyo xagel isithuthuthu ibhetrii-overcharges kunye noxinzelelo lwangaphakathi luphezulu, ngeli xesha ivalve ekhuselekileyo iya kuvala ngokwayo, ngoko akuyi kubakho igesi eyongezelelweyo iqokelela.INGCACISO YEMVELISO.
Akukho acid yasimahla:isahluli esikhethekileyo se-adsorb electrolyte, ngoko ke akukho acid yasimahla ngaphakathi kwebhetri ye-lead, emva koko ibhetri ye-vrla inokufakwa kwindawo eyahlukileyo.
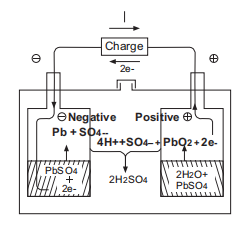
I-CHEMICAL REACTIONINVRLA BATTERY SA IYALANDELA
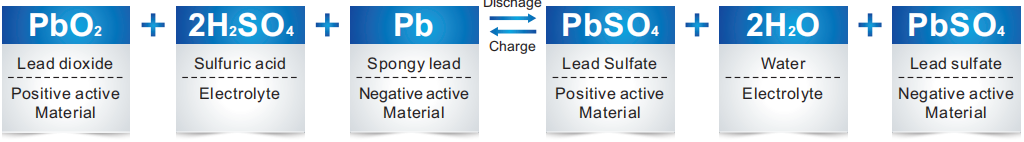
Ngelixa ibhetri yejeli ikhutshwa, ukuxinwa kwe-asidi ye-sulfuric kuyancipha ngokuthe ngcembe kwaye i-sulfate ye-lead yenziwa phantsi kwempendulo phakathi kwe-lead dioxide ye-electrode e-positive, i-spongy lead ye-electrode engalunganga kunye ne-asidi yesulfuric kwi-electrolyte.
Ngelixa utshaja, i-lead sulfate kwi-electrode efanelekileyo kunye ne-negative iguqulwa ibe yi-lead dioxide kunye ne-spongy lead, kwaye ngokuhlukana kwee-ion ze-sulfuric, ukuxinwa kwe-asidi ye-sulfuric kuya kwanda.
Ngexesha lokugqibela lokutshaja kwebhetri yeasidi ekhokelayo, amanzi asetyenziswa kukusabela kokuvela kwehydrogen.Ngoko ke ifuna imbuyekezo yamanzi.Ngokusetyenziswa kwe-spongy lead efumileyo, ikhawuleza isebenze neoksijini, elawula ngokufanelekileyo ukuhla kwamanzi.
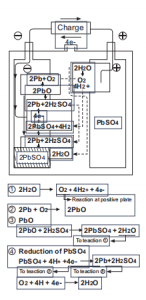
Kuyafana neebhetri zejeli zemveli ukusuka ekuqaleni kwentlawulo ukuya phambi kwenqanaba lokugqibela, kodwa xa ihlawulwe kakhulu kwaye kwixesha lokugqibela lokuhlawula, amandla ombane aya kuqalisa ukubola amanzi, i-electrode engalunganga iya kuba kwimeko yokukhutshwa. kuba ioksijini esuka kwipleyiti evumayo iphendula nge-spongy lead ye-negative plate kunye ne-sulfuric acid ye-electrolyte.Oko kuthintela i-hydrogen evolution kwiipleyiti ezimbi.Inxalenye ye-electrode engalunganga kwimeko yokukhupha iya kutshintsha ibe yi-spongy lead ngelixa itshaja.
Ubungakanani belothe ye-sponji eyenziwe ngokutshaja bulingana nobuninzi belothe yesulfate ngenxa yokufunxa ioksijini kwi-electrode eyakhayo, egcina ibhalansi ye-electrode engeyiyo, kwaye yenza kube lula ukutywinwa.12v 12ah gel ibhetri yeseli.Ukusabela emva kwenqanaba lokugqibela lentlawulo kunye nekhemikhali equation njengoko kulandelayo:
Umfanekiso wesi-3:Iintshukumo ukusuka ekuqaleni kweTyala ukuya phambi kwenqanaba lokugqibela
kutheni sisikhetha?
1. 100% Ukuhlolwa kwangaphambili kokunikezelwa ukuqinisekisa umgangatho ozinzileyo kunye nokusebenza okuthembekileyo.
2. I-Pb-Ca i-alloy yegridi ye-VRLA ipleyiti yebhetri, ilahleko yamanzi aphantsi, kunye nomgangatho ozinzileyo wezinga lokuzikhupha.
3. Ukuchasana okuphantsi kwangaphakathi, ukusebenza kakuhle kokukhupha izinga eliphezulu.
4. Uyilo lwe-electrolyte ekhukhulayo, i-electrolyte eyaneleyo, i-high-charge / over-discharge resistance.
5. Ukugqwesa ukusebenza kobushushu obuphezulu naphantsi, ubushushu obusebenzayo ukusuka kwi -25℃ ukuya kwi-50℃.
6. Yila ubomi benkonzo yokudada: iminyaka emi-3-5.
Ixesha lokuposa: Apr-07-2022

